![]()
McReynolds Constants and Retention Indices in GC
Retention Index: The selectivity of the chromatographic columns to analytes in GC is primarily determined by the stationary phase, because of the difference in the retention imposed by the stationary phase on different analytes. The polarities of the analyte and stationary phase determines the extent of retention of analytes. To systematically express the retention of an analyte on a given stationary phase (column) a measure called retention index is defined. Retention index places the retentive attributes of an analyte on a column in relation to the retention characteristic n-alkanes on that column.
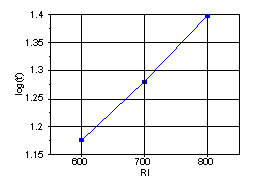
By definition the RI of a normal alkane is set to 100n, where n = number of C atoms of the alkane. It can be shown that they are related in forms (equivalent) shown below.
|
|
The values of a, b, a’ and b’ are determined by the column and the parameters used in the system. The retention index of an analyte eluting at t’, between two n-alkanes with number of carbon atoms n and N having corrected retention times t’n and t’N respectively will then be;
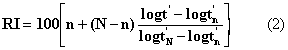
Thus RI measures the selectivity of the phase. Usually the analyte of which
the RI is sought, is bracketed between n- and N- C atom n-alkanes such that
N = n + 1 for accuracy. Any homologus series of compounds of a certain
functionality family will also exhibit the linear dependence as shown above
in (1) and (2).
McReynolds Constants
To compare the polarities of different stationary phases a standard stationary
phase has been defined, squalene, which is essentially a non-polar
phase.
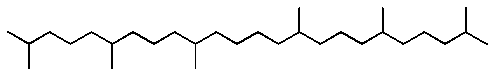
squalene
Squalene can exert Van Der Walls (VDW) type intermolecular attractive forces only. With squalene intermolecular forces as the base, all other attractive forces will be stronger in comparison. The retention of an analyte with some polar character on another stationary phase, compared to the analyte retention in squalene would measure the polar attractive forces between the stationary phase on the polar analyte. There are various types of intermolecular forces encountered between substances. They arise due to some polar character of the substances. Classifying the intermolecular forces, from a practical point of view with reference to the chemical moiety present in different classes of compounds, Probe compounds have been selected to represent the interaction of classes of compounds with similar intermolecular attractive strengths. The McReynolds constant for a given probe compound measure the degree of attractive interaction between probe compound and a stationary phase. The difference of the RI of the probe in the stationary phase and squalene is expressed as an index code. The magnitude of the index code gives a measure of the attractive interaction between the type of chemical moiety of the probe and the stationary phase.
Probe Compounds and Types of Molecular Interactions Measured:
| Probe Compound |
Index Code, |
Typical Characteristic Molecular Interaction |
| benzene | x’ |
pi - type (aromatic, olefinic) |
| 1-butanol | y’ |
electron attracting effect (alcohols, nitriles, acids, nitro compounds, chloro compounds) |
| 2-pentanone | z’ |
dipole - dipole (ketones, aldehydes, ethers, epoxides, amino) |
| 1-nitropropane | u’ |
electron donating effect (nitro, nitrile) |
| pyridine | s’ |
non bonding electron attraction (bases), H bonding capability |
D I = (Isp - Isqualene) for the probe compound.
Polarity of a Stationary Phase is given by,
![]()
McReynolds values can be used to compare different phases for their
similarity and dissimilarity and classify phases according to their relative
polarity. The value of D I = 0 - 100 non-polar,
100 - 400 moderately polar, >400 is highly polar. Also, the probe compound
indices would give the relative order of elution for similar
compounds.